
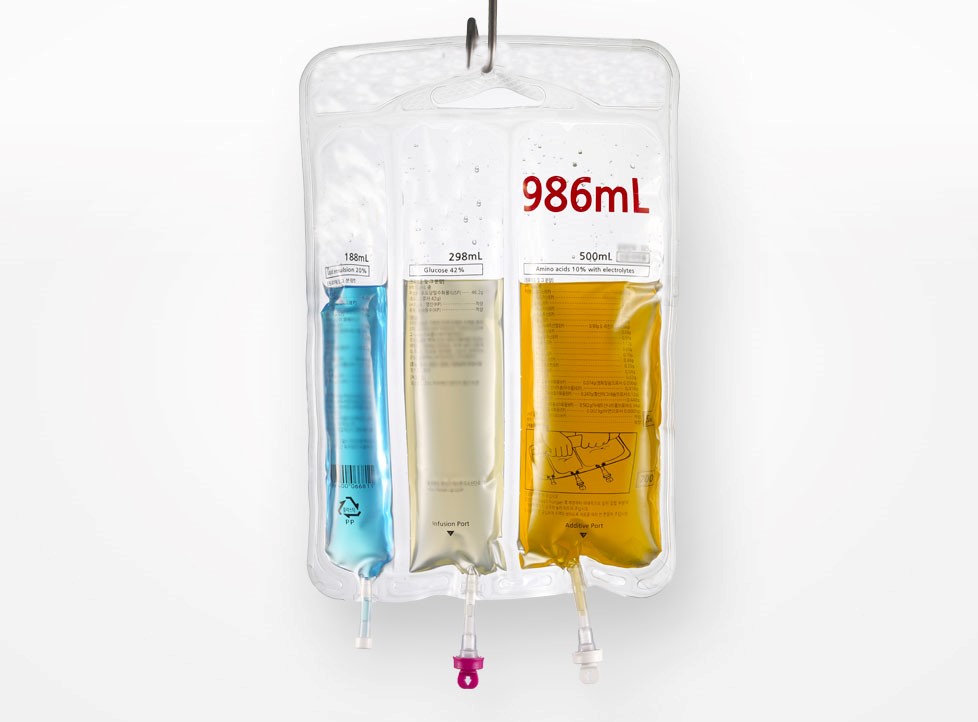
N'ime ụlọ ọrụ ọgwụ zuru ụwa ọnụ na-eto ngwa ngwa taa, ọgwụgwọ intravenous (IV), dị ka isi njikọ na ọgwụ ụlọ ọgwụ, etinyela ụkpụrụ dị elu na-enwetụbeghị ụdị ya maka nchekwa ọgwụ, nkwụsi ike, na nrụpụta nrụpụta. The Multi Chamber IV akpa, na ya pụrụ iche ngalaba imewe, nwere ike nweta ozugbo agwakọta ọgwụ na ihe mgbaze, ukwuu mma ọgwụ ziri ezi na mma. Ọ bụrụla ụdị nkwakọ ngwaahịa kachasị amasị maka nkwadebe siri ike dị ka nri parenteral, ọgwụ kemoterapi, ọgwụ nje, wdg. Otú ọ dị, mmepụta nke ngwaahịa ndị dị otú ahụ na-achọ ihe ndị dị oke mkpa maka teknụzụ akụrụngwa, gburugburu ebe dị ọcha, na nnabata. Naanị ndị na-enye ọrụ injinia nwere nnukwu mkpokọta teknụzụ yana ahụmịhe ọrụ zuru ụwa ọnụ nwere ike ịnye azịza a pụrụ ịdabere na ya n'ezie.
Dị ka onye ndu mba ụwa na ngalaba nke injinia ahụike, IVEN Pharmatech Engineering, nke nwere ahụmịhe karịrị afọ 30 na ụlọ ọrụ ọgwụ, na-agba mbọ inye ndị ahịa ụwa ọrụ injinia otu nkwụsịtụ site na imepụta usoro, ntinye akụrụngwa na asambodo nnabata. AnyịMulti Chamber IV akpa Production LineỌ bụghị naanị na ọ na-ejikọta teknụzụ akpaaka na-egbutu ọnụ, kamakwa ọ nwere uru bụ isi nke 100% nrube isi na ụkpụrụ mba ụwa dị ka EU GMP na US FDA cGMP, na-enyere ụlọ ọrụ na-emepụta ọgwụ aka nke ọma ịmepụta ngwaahịa ndị bara uru dị elu ma jide ohere ahịa ụwa.
Multi Chamber IV Akpa Intelligent Production Line: Na-akọwapụta oke n'etiti arụmọrụ na nchekwa
A haziri ahịrị mmepụta akpa infusion nke ọtụtụ ụlọ nke IVEN iji zute ihe ịma aka nke mmepụta mmepụta ihe dị mgbagwoju anya. Site n'ụyọkọ teknụzụ ọhụrụ anọ, ọ na-enyere ndị ahịa aka imebi n'ọgba aghara mmepụta ọdịnala:
1. Multi chamber synchronous ịkpụzi na kpọmkwem ndochi teknụzụ
Akpa otu ọnụ ụlọ ọdịnala na-adabere na nzọụkwụ ngwakọta mpụga, nke na-ebute ihe ize ndụ nke mmetọ obe na adịghị arụ ọrụ. IVEN na-anakwere usoro ihe nkiri nwere akụkụ atọ nke nwere akụkụ atọ. Site n'ụdị dị elu dị elu na njikwa gradient okpomọkụ, enwere ike ịmepụta ụlọ 2-4 nọọrọ onwe ha n'otu stampụ, na ike nkebi nke karịrị 50N / 15mm n'etiti ụlọ, na-eme ka nkwụsị efu efu n'oge njem na nchekwa. Usoro ndochi ahụ na-ewebata mgbapụta igwe na-ejuputa ọtụtụ ọwa nke magnetik levitation linear moto na-ebugharị, yana opekempe ndochi nke ± 0.5%, na-akwado mgbanwe dịgasị iche iche site na 1mL ruo 5000mL, na-ekwekọ n'ụzọ zuru oke na mkpa nkwakọ ngwaahịa nke mmiri viscosity dị iche iche dị ka ngwọta nri na ọgwụ chemotherapy.
2. Sistemụ njikọ ekpochi akpọchi kpamkpam
Iji dozie nsogbu njikwa microbial na akpa agwakọta ọtụtụ ụlọ, IVEN ewepụtala ngwaọrụ ịgbalite SafeLink ™ Aseptik. Ngwa ahụ na-anabata ihe eji eme ihe na-eme ka ike gwụ nke laser, jikọtara ya na usoro na-ebute nrụgide n'ibu. Ndị ọrụ ahụike kwesịrị iji otu aka afanye iji nweta nkwurịta okwu na-adịghị mma n'etiti ụlọ, na-ezere ihe ize ndụ nke irighiri iko nke nwere ike ịmalite site na valvụ mpịachi ọdịnala. Mgbe nkwenye nke ndị ọzọ gachara, arụmọrụ akara nke njikọ arụ ọrụ na-ezute ọkọlọtọ ASTM F2338-09, yana ohere nke mwakpo microbial erughị 10 ⁻⁶.
3. Nleba anya nleba anya na usoro traceability ọgụgụ isi
The mmepụta ahịrị integrates AI X-ray dual-mode usoro nchọpụta, nke synchronously achọpụta ihe nkiri ntụpọ, na-ejuputa mmiri mmiri larịị deviations, na ụlọ akara iguzosi ike n'ezi ihe site na elu-mkpebi CCD ese foto na micro focus X-ray imaging. Algọridim mmụta mmụta miri emi nwere ike ịchọpụta ntụpọ pinhole na-akpaghị aka na ọkwa 0.1mm, yana ọnụọgụ nchọpụta ụgha nke na-erughị 0.01%. N'otu oge ahụ, a na-akụnye akpa infusion nke ọ bụla na mgbawa RFID iji nweta traceability zuru oke site na batches akụrụngwa, mmepụta ihe na-ekesa okpomọkụ, na-ezute ihe ndị chọrọ serialization nke FDA DSCSA (Drug Supply Chain Safety Act).
4. Energy ịzọpụta na-aga n'ihu sterilization ngwọta
The omenala intermittent sterilization kabinet nwere ihe mgbu isi nri ike dị elu na ogologo okirikiri. IVEN na ndị mmekọ ya na Jamanị ejikọtala ọnụ emepụtala sistemu Rotary Steam in Place (SIP), nke na-anakwere imewe ụlọ elu na-agbagharị agbagharị iji mepụta ọgba aghara n'ime ụlọ a na-ekpo ọkụ ọkụ. Ọ nwere ike mezue sterilization n'ime 15 nkeji na 121 ℃, na-azọpụta 35% ume tụnyere omenala ụzọ. Ejiri usoro a nwere njikwa B&R PLC nke mebere onwe ya, nke nwere ike ịdekọ ma chekwaa data nkesa okpomọkụ nke batch ọ bụla (F ₀ uru ≥ 15), wee mepụta ndekọ batrị eletrọnịkị na-akpaghị aka nke kwekọrọ na 21 CFR Nkebi 11.
Nkwenye nke IVEN: netwọk ọrụ zuru ụwa ọnụ gbadoro ụkwụ na ọganihu ndị ahịa
Anyị maara nke ọma na akụrụngwa klaasị mbụ kwesịrị ijikọ na ọrụ ọkwa mbụ.IVEN eguzobewo ụlọ ọrụ teknụzụ na mba 12 n'ụwa niile, na-enye nyocha 7 × 24 awa anya na nkwado nzaghachi 48 na saịtị. Ndị otu anyị nwere ike ịnye ọrụ azụmaahịa ahaziri ahazi dabere na ụkpụrụ dị iche iche na mpaghara dị iche iche.
N'oge ọgwụgwọ ziri ezi na ọgwụ ahaziri onwe ya, akpa intravenous intravenous nke ọtụtụ ụlọ na-emezigharị oke ọgwụgwọ parenteral. IVEN Pharmatech Engineering na-ewuli akwa ga-eme n'ọdịnihu maka ụlọ ọrụ na-emepụta ọgwụ zuru ụwa ọnụ na ọkachamara injinịa ya pụtara ìhè na nchụso kachasị nke nnabata. Ma ọ bụ ọrụ ọhụrụ ma ọ bụ nkwalite ikike, ahịrị mmepụta anyị nwere ọgụgụ isi ga-abụ onye mmekọ ntụkwasị obi gị kachasị.
Kpọtụrụ ndị IVENndị otu ọkachamara ozugbo maka ngwọta ahaziri iche na akụkọ ịga nke ọma zuru ụwa ọnụ!
Oge nzipu: Mee-27-2025


