Sistemu bioprocess (isi bioprocess nke mgbago na mgbada)
IVEN na-enye ngwaahịa na ọrụ na ụlọ ọrụ biopharmaceutical ụwa na ụlọ ọrụ nyocha, ma na-enye azịza injinia ahaziri ahazi dị ka mkpa onye ọrụ si dị na ụlọ ọrụ biopharmaceutical, nke a na-eji n'ọhịa nke ọgwụ protein recombinant, ọgwụ mgbochi mmadụ, ọgwụ mgbochi na ngwaahịa ọbara.
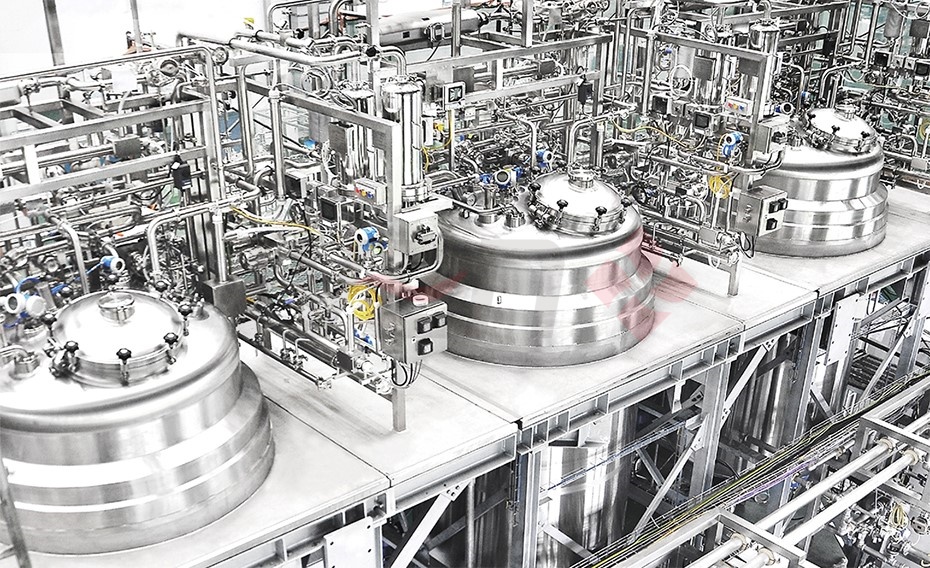
Lekwasị anya n'inye ụlọ ọrụ biopharmaceutical zuru ezu biopharmaceutical upstream na downstream usoro akụrụngwa na isi usoro metụtara usoro injinia ngwọta, gụnyere: usoro teknụzụ ndụmọdụ ọrụ, media nkwadebe na nkesa ngwọta, gbaa ụka usoro / bioreactors, chromatography usoro , Nkwadebe ngwọta ndochi ngwọta, ngwaahịa nkọwa na owuwe ihe ubi ngwọta, buffer nkwadebe na nkesa ngwọta, miri filtration usoro ngwọta usoro modul, miri emi filtration usoro ngwọta usoro, usoro ihe ngwọta nkesa, chromatography usoro. centrifugal usoro modul ngwọta, nje na-etipịa usoro ngwọta, ngwaahịa ngwọta nkwakọ usoro ngwọta, wdg IVEN na-enye biopharmaceutical ụlọ ọrụ na a zuru ezu nke ahaziri n'ozuzu engineering ngwọta si ọgwụ nnyocha na mmepe, pilot ule na mmepụta, na-enyere ndị ahịa nweta elu-ọkọlọtọ na oru oma usoro eruba. Ngwaahịa na-agbaso ISO9001, ASME BPE na ụkpụrụ akụrụngwa biopharmaceutical ndị ọzọ, ma nwee ike inye ụlọ ọrụ ọrụ na aro zuru oke na nhazi usoro, owuwu injinia, nhọrọ akụrụngwa, njikwa mmepụta na nkwenye.




